Ammonium is formed by the addition a hydrogen atom to ammonia (NH3). This forms a positive cation with a +1 charge on the nitrogen atom. As a result, NH4+ is weakly acidic, wishing to exchange a hydrogen with a lone pair of electrons to achieve a neutral charge. Ammonium is also capable of forming a wide variety of salts. However, some of these are noted to be quite explosive.
To learn more about the polarity and lewis dot structure of NH3, feel free to check out this article and this brief, respectively.
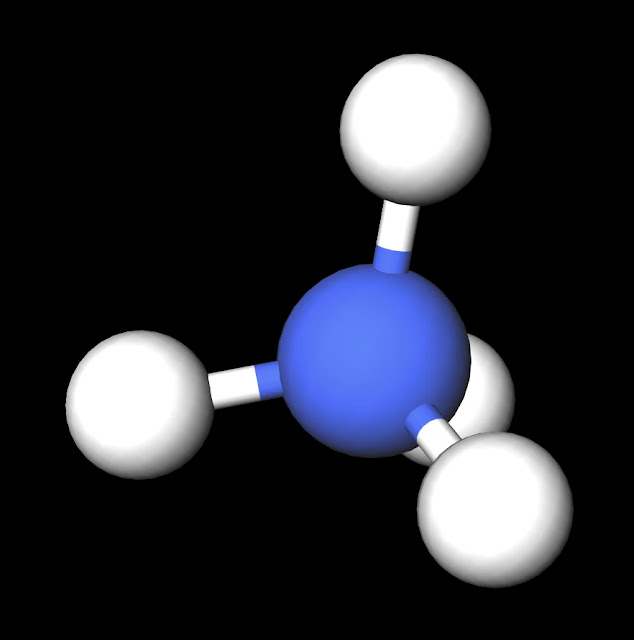 |
| NH4+ Ball and Stick Model. Created with MolView. |
One of the most recognizable functional groups within biology amine group (NH, NH2 or NH3). This is an important feature of amino acids, allowing primary structures to form. Moreover, ammonium ions are a byproduct of metabolism within the human body. While many species convert this product to urea for disposal, there are certain fish that release it directly into the surrounding water. NH4+ is also an important nitrogen source for plants; however, it is toxic at high concentrations and therefore cannot be relied upon solely.
pg ยอดเยี่ยมเกมออนไลน์สล็อตบนมือถือแบบใหม่ปัจจุบันของโลกสมัครเล่น PG SLOT วันนี้ไม่มีเบื่อไม่ซ้ำซากในแบบการเล่นเดิมๆอีกต่อไปเป็นเกมสล็อตที่แจ๊คพอตแตกหลายครั้งที่สุดลองเลย
ReplyDelete