 |
| Created by MakeTheBrainHappy. |
The Lewis Dot Structure for NH3 (Ammonia) is shown above. You could also represent the bonds as dots between the two atoms, but this may be confused with the lone pair electrons on the nitrogen.
Each atom in the bond has a full valence shell, with nitrogen having access to eight electrons and each hydrogen having access to two (learn why hydrogen only needs two). The covalent bonds between the N and the H are similar to the ones formed between two Hs because the relatively small difference in electronegativity between carbon and hydrogen.
Nitrogen requires a lone pair of electrons to complete the structure and provide five electrons as recommended by nitrogen's original elemental valence shell which contains the same number of electrons. It is difficult to ascertain the exact 3D structure, but it follows a general tetrahedron structure shown below. Due to electron-electron repulsion in the lone pair, the angles between the remaining hydrogens is slightly less than 109.5˚: 107.5˚. This places nitrogen into the category of "trigonal pyramidal" geometric configurations.
Ammonia has a general tetrahedron structure in 3D space similar to methane. Source
Ammonia is related to ammonium (NH4+), whose lewis structure is shown here. Ammonia is generally a base. It is a type of nitrogenous waste especially prevalent in aquatic organisms. It is considered a hazard toward human health and can have the effects on the neural system as shown in the diagram below.
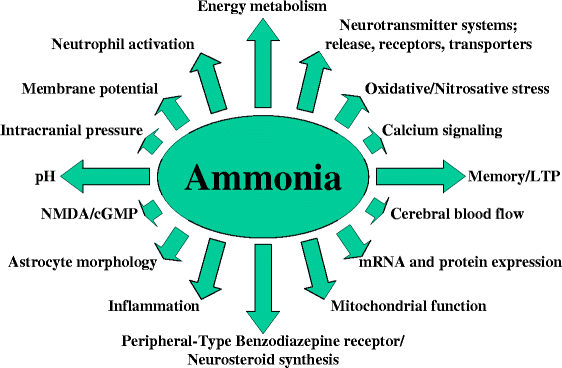 |
| Source. |
It is also one of the few compounds which can undergo hydrogen bonding due to its polar nature. Due to the strength of these hydrogen bonds, water has a relatively high melting and boiling point, although they are not as high as network covalent solids. Those are bonded by intramolecular forces which involve the actual sharing of electrons vs. partial dipole forces in hydrogen bonds. There are only three types of bonds which can hydrogen bond. These are N-H, O-H, and F-H bonds due to the large electronegativity differences between the molecules. In the H2O article, ammonia was briefly mentioned as a compound with these properties. These properties contribute to NO3s relatively high boiling point of -33.3˚C and high solubility in water.
Sources:

Thank you
ReplyDelete